TAVO
TAVO™ + Electroporation (EP) Gene Delivery
We are developing cytokine-based intratumoral immunotherapies to stimulate the body’s immune system to target and attack cancer. We have built a deep clinical pipeline utilizing our primary technology, TAVO™ (tavokinogene telseplasmid) as a potential treatment for multiple cancer indications either as a monotherapy or in combination with leading checkpoint inhibitors. TAVO has the potential to become the first approved therapeutic to address a great unmet medical need: anti-PD-1 non-responders.
TAVO is DNA-based interleukin-12 (IL-12), a naturally occurring protein in the body with immune-stimulating functions. We believe TAVO is administered directly into the tumor using our proprietary electroporation (EP) gene delivery system, which employs a series of momentary energy pulses. Those pulses are designed to increase the permeability of the cell membrane and facilitate uptake of IL-12 coded DNA into cells. This non-invasive method is easy to perform and avoids systemic toxicity issues historically associated with IL-12 usage.
Clinical studies have demonstrated that TAVO induces local expression of IL-12, converting immunologically suppressed “cold tumors” into T-cell inflamed “hot tumors” which is fundamental to generating objective responses in both treated and untreated distant tumors.
TAVO is being studied in multiple clinical trials, including a registration-directed pivotal Phase 2 trial in metastatic melanoma and two Phase 2 trials in triple negative breast cancer (TNBC) and head and neck cancer. Results from recently completed clinical studies of TAVO have demonstrated a local immune response, and subsequently, a systemic effect as either a monotherapy or combination treatment approach.
OncoSec’s TAVO: Learn From Physicians and Patients How It Works
OncoSec’s TAVO™ plus Electroporation Gene Delivery: Learn From Physicians and Patients How It Works
Key Highlights Include
How It’s Designed to Work
Roll over images to reveal how TAVO is designed to work.

Step 1
Cancer is identified in the body.

Step 2
DNA-based interleukin-12 (IL-12), a naturally occurring protein, is injected directly into the tumor.

Step 3
The applicator supplies a sequence of short-duration electrical pulses through a series of needles.
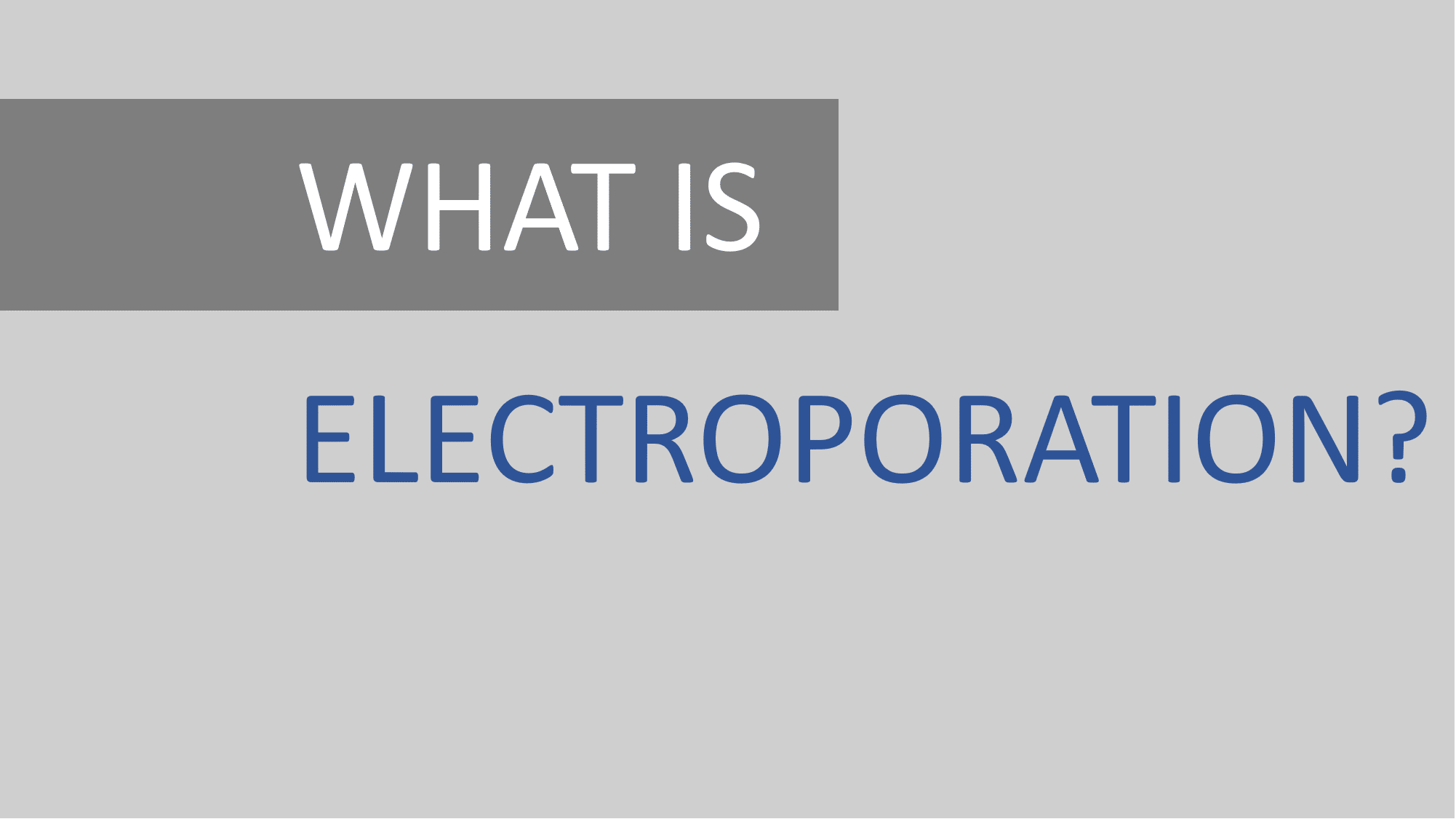
Step 4
Electrical pulses result in increased permeability of the cell membrane, allowing DNA-based IL-12 to enter.

Step 5
DNA-based IL-12 is expressed in the local tumor microenvironment.
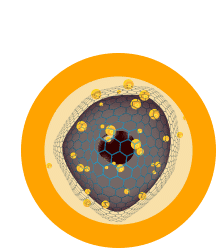
Step 6
Immune cells are educated to recognize the patient’s cancer.

Step 7
Immune cells are educated to recognize the patient’s cancer.
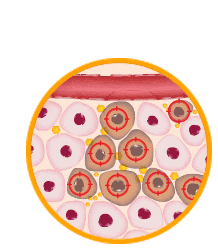
Step 8
Educated immune cells identify and attack tumors throughout the body.
